Photos / Sounds
What
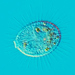
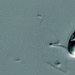
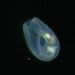
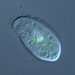
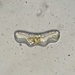
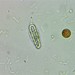
Peranema pleururumObserver
peptolabDescription
Pseudoperanema (Peranema) pleururum Skuja, 1948. P. pleururum (Skuja 1948) J.Larsen , a colorless euglenid species from the shoreline benthos of the freshwater glacial kettle hole Kellis Pond. Imaged in Nomarski DIC on Olympus BH2S using SPlan 100 1.25 oil immersion objective plus variable phone cropping on Samsung Galaxy S9+. The cells measure 100 um in length and have a single thick emergent flagellum which undulates at the tip and is as long as the plump body. There is a second recurrent flagellum in a groove (see pictures). The pellicle has prominent sigmoidal striations. The posterior end is acutely pointed. There are two thick ingestion rods. There is a contractile vacuole adjacent to the vestibule. There is a large central oval nucleus with a large oval nucleolus. Jára Kubín has pointed out that there is a second recurrent flagellum in a groove. This along with the pellicular striations, the two thick ingestion rods, and the asymmetrical pointed posterior makes this Pseudoperanema pleururum (1).
Schroeckh et al 2003 redescribed Peranema (Pseudoperanema) pleururum Skuja, 1948. "Description: Gliding, metabolic euglenid, cells 48– 140 µm long and 20–24 µm wide. Cell slightly spindle-shaped when gliding, but metabolic and more expanded when squirming. The cell is flattened and may be folded or twisted; the ventral side of cell has a wide convex gutter. The cell tapers anteriorly and the posterior end of the cell is pointed – sometimes with two points. The pellicle has strong longitudinal ridges (13 ridges per 10 µm). The anterior flagellum is about 1.0 cell length, and the posterior flagellum is about 0.75 cell length and lies in the ventral groove which runs along the longitudinal hollow gutter. The ingestion apparatus has two strongly developed rods. Each rod is anteriorly swollen. A well-developed arc structure connects to the rods. A contractile vacuole was observed in the anterior quarter of the cell very close to the reservoir. Cells contain many roundish paramylon grains. Extrusomes were not seen" (1).
"The most similar species are P. inflexum and P. trichophorum. Peranema pleururum can be distinguished by its size from P. inflexum by its hollowed / flattened appearance, from P. trichophorum and P. inflexum and by the well-defined cortical ridges, and from P. trichophorum by the lack of extrusomes. We believe that P. pleururum has previously been described from Australia by Playfair (1921) as Figure 8 under the name P. cuneatum. Peranema pleururum has been described from northern Europe, Australia and China (Playfair, 1921; Skuja, 1948; Shi, 1999). This species has a worldwide distribution" (1).
Duangjan et al described Peranema (Pseudoperanema) pleururum Skuja from northern Thailand; their population was smaller than the range described by Schroeckh 2003: "Cells with euglenoid movement, 38.8–75.0 µm long, 12.5–25.0 µm wide, wide lancet-shaped or spindle-shaped, slightly flattened, sometimes twisted; anterior end elongated, oblique; posterior end narrow, incised end, with lateral appendix; groove narrow on ventral side; pellicle quite thick, longitudinally striated, striation interrupted; anterior flagellum thick, longer than cell, posterior flagellum thin, lying in groove. Sites: Chiang Mai – fish pond (UM), garden pond (AG). General occurrence: rare, small rivers, ponds, puddles; reported from Asia: India (Gupta 2012); Europe: Latvia, Ukraine, Russia; Australia (Schroeckh et al. 2003)" (2).
The taxonomy of Jenningsia, Peranema, Peranemopisis, and Pseudoperanema has been everchangeing, confusing and convoluted partly by some generic names being assigned to other types of organisms and also by confusion regarding the presence or absence of an often difficult to see recurrent second flagellum. Kubin et al 2024 revised the genus Pseudoperanema and wrote: "Pseudoperanema pleururum (Skuja) J.Larsen Skuja (1948) (Pseudoperanema pleururum (Skuja) J.Larsen, Nordic J. Bot. 7: 600. 1987. Basionym: Peranema pleururum Skuja, Symb. Bot. Upsal. 9(3): 232, = Peranema caudatum Skuja, Nova Acta Regiae Soc. Sci. Upsal., ser. 4, 16(3): 253, Tab. 45: Fig. 11. 1956, syn. nov. Note: Skuja (1956) described Peranema caudatum as differing from Peranema pleururum Skuja (Pseudoperanema pleururum (Skuja) J.Larsen) by its concave cross-section and larger size (90–135 µm versus 62–75 µm in P. pleururum). Nevertheless, Popova and Safonova (1976) described the size of P. pleururum as reaching 62–75(-120) µm in length without mentioning the supposedly larger P. caudatum and, similarly, Starmach (1983) and Schroeckh et al. (2003) preferred to extend the morphological limits of P. pleururum, accepting the range of 48–140 µm, rather than segregating P. caudatum, as no other morphological differences were observed. The synonymization of P. pleururum and P. caudatum was suggested by Schroeckh et al. (2003) but not formally effected. In agreement with their taxonomic opinion, the formal synonymy is proposed here" (3).
-
Free-living heterotrophic euglenids from freshwater sites in mainland Australia. Sabrina Schroeckh, Won Je Lee & David J. Patterson. Hydrobiologia 493: 131–166, 2003.
Polish Botanical Journal 62(1): xx–xx, 2017 DOI: 10.1515/pbj-2017-0005
Checklist of colourless euglenoids of the Czech Republic, with several taxonomic additions Jaroslav Kubín, Josef Jura´nˇ , Jan Kuˇcera Protist 175 (2024) 126045
Photos / Sounds
What
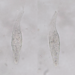
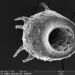
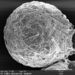
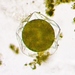
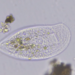
Genus ActinobolinaObserver
davidfbirdDescription
Size: 90 X 65 µm, rigid spines are 40 µm. There's a thick inner layer of flagella or cilia. The cell is capable of rapid locomotion - see third gif. I'm flummoxed - is it even a heliozoan? @bdstaylor, any ideas?
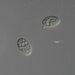
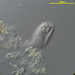
Photos / Sounds
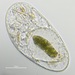
Observer
peptolabDescription
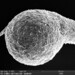
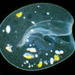
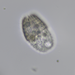
Cryptopharynx setigerus KAHL, 1926, a psammobiontic karyorelectean ciliate from the fine sand sulfidic intertidal benthos of the marina at Moneybogue Bay in Westhamtpon Beach. The cell measures 80 um.
Here is a translation of Kahl's original 1928 description of the species: Cryptopharynx setigerus KAHL, 1926 Size 40-100 um Slim oval (3:1). Front third protruding to the left (1/3 width of the body). Ventral surface flat, dorsal with hump leaving the front third and a closed edge free. Ventral surface loosely ciliate in furrowed rows. The insertions are arranged in transverse rows that cross the longitudinal rows in a rhombic manner. Intermediate stripes rib-shaped. The furrows run spirally (see illustration) and notch the edge; here each notch bears on the dorsal surface an erect stinger. The rows wrap around the very left. Mouth lying in front; it is oval on a protruding cone, appears open, shows short rods that are difficult to see on the edge, but there are clearly visible rods inside; the cilia of the perioral row hit over the edge, no additional adorable ciliation. Dorsal hump is yellowish and granulated, with gelatinous coating on the outside. Two nuclei with one or two micronuclei, exactly like those of Loxodes. Contractile vacuole at the back of the hump, small, often difficult to see, often behind the middle left also a vacuole (whether contractile?); Anus at the back of the hump. Movement very sluggish, very flexible. Halobiont, sapropel. Eats almost exclusively Rhodobacteria.
The Infraciliature of Cryptopharynx setigerus KAHL, 1928 and Apocryptopharynx hippocampoides nov. gen., nov. spec. (Ciliophora, Karyorelictea), with an Account on Evolution in Loxodid Ciliates. WILHELM FOISSNER. Arch. Protistenkd. 146 (1995/96): 309-327
What
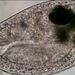
Genus ChroococcusObserver
crseaquistDescription
This sample came from scrapping algae up from puddles on the floor, 2024/09/11.
Thank you to the Tice Lab for allowing me to use their microscope.
Photos / Sounds
Observer
peptolabDescription
My recent sample from the shoreline benthos of freshwater glacial kettle hole Kellis Pond is rich in these tiny testate amoebae which average 21.6 um in diameter. The test is usually stained brown, is not invaginated and is flattened hemispherical in profile, has an aperture around 4/5 of the test diameter, and is finely textured with uniformly spaced and sized grains on the aboral surface. I have yet to observe pseudopodia in a couple hundred cells, but in one cell I saw what looks like a broad lobose pseudopod. The resident rounded amoebae have a large round macronucleus with a large central nucleolus and a single contractile vacuole. I note amoebae which have left their test ( or have yet to form it).
Imaged in Nomarski DIC on Olympus BH2S using SPlan 100 1.25 oil immersion objective plus variable phone camera cropping on Samsung Galaxy S9+.
Thanks to Ferry Siemensma for identifying this as Pyxidicula operculata.
From Ferry Siemensma's Microworld: https://arcella.nl/pyxidicula-operculata/
Pyxidicula operculata, (Agardh, 1827) Ehrenberg, 1838
Diagnosis: shell discoid or hemispherical, composed of organic material, which varies in color, being transparent or light yellow in young individuals and becoming darker with age to be either brown or red-brown; aperture almost as wide as the total diameter of the shell; shell uniform in outline with a slight thickening around the margin; this thickening is a slightly depressed rim, which is recurved to give a small lip; shell covered by regularly spaced pits or pores, but the surface is smooth on the apertural margin; a single vesicular nucleus with a centric nucleolus; pseudopodia short, lobose or digitate.
Dimensions: Diameter 13.6-31 µm.
Ecology: On aquatic vegetation. Very common, but easily overlooked.
Remarks: Portions of the broken rim show that it is a thin extension of the shell wall, which is constructed of a single layer of regular, interlocking alveoli. These alveoli are rectangular in vertical section with a depth of about 0.3 µm and have a solid content and thin walls. The contents of the alveoli did not permit embedding resin to penetrate the whole wall, and as a consequence sectioned material gave poor results. Nevertheless, sufficient information is available to reconstruct diagrammatically a cross-section through the wall. Each alveolus is surrounded by a single membrane-like structure, which is referred to as the shell matrix, and at the outer surface the shell wall is bilaminar. See for further information Ogden (1987).
Photos / Sounds
Observer
crseaquistDescription
This sample came from scrapping algae up from puddles on the floor, 2024/09/11.
Thank you to the Tice Lab for allowing me to use their microscope.
Photos / Sounds
What
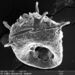
Netzelia tuberculataObserver
bdstaylorDescription
Some specimens have the distinctive tubercular shell that gives the species its name. Others, like this one, have fairly smooth shells, incorporating diatoms, scales of heliozoans and other siliceous materials.
Photos / Sounds
What
Nematodes (Phylum Nematoda)Observer
mnold1Description
Garden cucumber leaf. Looking like a flight over an agricultural landscape, this patchwork pattern is characteristic of a mosaic virus infection. An inexpensive stereo microscope was used for a closer look at the green and brown rectangles. Trichomes were an interesting target, appearing more white and opaque in the brown areas versus more translucent in the live, green areas. A square cm of leaf tissue was put on glass slide with water and a coverslip and observed under a compound microscope. Surprisingly, nematodes appeared to be wriggling out of the tissue (at least a dozen or more were observed), suggesting this may be a nematode disease rather than CMV (?). Note, this leaf was trellised and had no ground contact.
Photos / Sounds
What
Notosolenus ostiumObserver
peptolabDescription
A petalomonad flagellate from the benthos of the channel connecting estuary Napeague Bay with Fresh Pond. The salinity of the channel is 30%. Imaged in Nomarski DIC on Olympus BH2 using SPlan 40 0.70 objective plus variable phone camera cropping on Samsung Galaxy S9+. The consensus is that this is a species of Notosolenus. Thanks to Alastair Simpson for confirming that this is Notosolenus ostium.
The cell measures 50 um in length. Originally I did think I was looking at N. ostium Larsen and Patterson 1990. The overall cell shape, size and prominent mouth or ostium are consistent with this as are the delicate ingestion organelle, the position and shape of the nucleus, and the two flagella- the anterior locomotive one as long as the cell and the shorter one only 0.2 times cell length which attaches to the substrate. What troubled me about this diagnosis is I can see no dorsal or ventral grooves. David Patterson feels that these may be less apparent in well-fed individuals (personal communication). Also, there seems to be a double row of round extrusomes adjacent to the delicate rods of the ingestion apparatus which is not described for this species or, I thought, for this genus.
But....thanks are due to Alastair Simpson for pointing out that my observation has extrusomes similar to those he and Won Je Lee 2014 demonstrated in Notosolenus urceolatus Larsen and Patterson, 1990: "a previously undocumented type of large, globular extrusome is present instead of the tubular extrusomes characteristic of Euglenozoa" (1). Lee and Simpson 2014 also noted " refractile granules lying between the left vestibule margin and the anterior of the cell, often along ridges" (3). However, N. urcelolatus differs from my observation in it's size: variously reported as 11–18.8 um (1) and 15-22 um (2) versus 50 um for this observation and 27-56 um for U. ostium (2).
Also, the posterior flagellum of N. urceolatus is reported as 3/4 cell length (3) or 0.2-0.5 um cell length (1) whereas this structure in my observation is 0.2 cell length and in N. ostium it is reported as 0.2-0.6 cell length.
Thus, based mainly on shape, notably in N. urceolatus the
anterior end formed a short narrow neck around the flagellar canal that was bent to the right (1), cell size, and to a lesser extent posterior flagellum length, I feel that my observation is more consistent with N. ostium. Alastair's kind communication regarding the globular extrusomes found in at least one Notosolenus species dispells my concerns at least about genus identification. I note that Lee and Simpson described other workers finding similar putative extrusome structures in two other Notosolenus species (1). I provide below original descriptions of both N. ostium and N. urceolatus.
" Notosolenus ostium Larsen and Patterson , 1990 Description. Cell outline elongate ovate, 27-56 um long, 15-24 mm wide, the ratio of length to width is 1.5 to 3.2. Dorso-ventrally flattened, dorsally with a median longitudinal groove, and ventrally a wide groove and four fine stripes. With a small obliquely oriented ingestion organelle with two fine rods near the anterior. The majority of the cells have a rounded posterior end but some cells have a slightly pointed posterior end. The reservoir is anteriorly situated in the right side of the cell and the nucleus in the left side. Two flagella of unequal length; the anterior flagellum is as long as the cell, held forward in gliding cells. The posterior flagellum is about 0.2-0.6 times the length of the cell. The organism contained eukaryotic algal material up to 8 mm long. Moves by smooth gliding with the anterior flagellum. Common in late culture. Description based on observations of 30 cells" (2).
"Remarks. Previously reported lengths of cells from marine sites (subtropical and tropical Australia, Brazil, Fiji, Hawaii and Panama) range from 24 to 40 mm (Larsen and Patterson, 1990; Ekebom et al., 1996; Patterson and Simpson, 1996 ). Our observations extend the size range. We observed two cells measuring 43 and 56 um which may be assignable to N. ostium. Notosolenus ostium is easily distinguished from other species of Notosolenus by its deep dorsal groove and visible ingestion organelle, which has not been seen in other species of the genus except N. triangularis Larsen and Patterson, 1990. Notosolenus ostium is similar to N. lagenos Skuja, 1948 in length and general appearance and in having a very short recurrent flagellum, but N. ostium can be distinguished by its wide grooves on both faces of the cell" (2).
"Notosolenus urceolatus Larsen and Patterson, 1990. Description. Cell pitcher-shaped, 15- 22µm long, 9- 14µm wide, anteriorly flagella emerge from short protrusion or neck which may or may not lie at a slight angle; posterior broadly obtuse. Ventrally with a shallow median groove and fine distant stripes, dorsally with three ridges. Reservoir in the right hand side of the cell, with refractile granules lying between its left margin and the anterior of the cell, often along ridges. Anterior flagellum slightly longer than the cell, posterior flagellum about 3/4 of the cell length. Nucleus in the left hand side of the cell. Found. Bowling Green Bay, Queensland; Ilha do Fundao, Rio de Janeiro.
Remarks. Notosolenus urceolatus is distinguished by the dorsal ridges which are rare in the genus. N. chelonides Skuja, 1939 has dorsal keels but they are much more conspicuous than in the present species. N. chelonides is twice as large as N. urceolatus.
N. esulcis also has dorsal grooves, but does not have the ventral groove and is more acute anteriorly" (3).
-
Morphological and Molecular Characterisation of Notosolenus urceolatus Larsen and Patterson 1990, a Member of an Understudied Deep-branching Euglenid Group (Petalomonads)
Won Je Lee & Alastair G. B. Simpson. Journal of Eukaryotic Microbiology 2014, 61, 463–479 - Heterotrophic flagellates (Protista) from marine sediments of Botany Bay, Australia WON JE LEE and DAVID J. PATTERSON. Journal of Natural History, 2000, 34, 483-562
-
Some flagellates (Protista) from tropical marine sediments
JACOB LARSEN and DAVID J. PATTERSON. J. Nat. Hist. 1990, 24, 801- 937.
Photos / Sounds
What
Peritromus kahliObserver
peptolabDescription
Peritromus kahli Villeneuve-Brachon, 1940 from the sulfidic benthos of a tiny tidal pool at the edge of the salt marsh abutting estuary Acabonac Harbor at Landing Lane. The cell measures 100 um in length. Imaged in Nomarski DIC on Olympus BH2S using SPlan 40 0.70 objective plus variable phone camera cropping on Samsung Galaxy 9+. We can see the characteristic contraction of the cell when disturbed. It is only then that we can just make out, at the light microscopic level, a coating on the dorsal surface of peculiar "chalice-like" or "golf tee-like" structures which are specific to P. kahli (1,2).
"When stimulated (mechanically, chemically), it rapidly contracts in 0.6-0.7 s. The anterior and posterior regions of the rim of the dorsal surface turn abruptly on the ventral surface and enfold it. In this way, the ciliate reduces its dimensions to - 1/3 and assumes a roughly spherical shape in which only the dorsal surface is exposed. Considering that contraction is induced by external stimuli, and that only the dorsal surface is exposed in contracted P. kahli, the presence of those external structures may have an impact on predator-prey interaction, thus providing a defensive function. The dorso-ventral differentiation that certainly influences the behavior of P. kahli (e.g. preference for crawling and thigmotaxis) may have been selected as an adaptation to the constraints of the interstitial habitat" (1).
"General morphology. Peritromus kahli is dorso-ventrally flattened with a dorsal irregular hump surrounded by a sort of flat rim. It is oval in shape, both ends are rounded. In vivo sizes: 116 (80-150) X 68.3 (44-92) um. The entire, flattened ventral surface is covered by 29 (25-33) densely ciliated kineties, very close to each other. There are two dorsal kineties: the more internal one (IDK), consisting of cilia, 5-6 um apart, borders the hump; and the external dorsal kinety (EDK) runs along the very limit between the dorsal and the ventral surfaces with cilia 3-4 um apart. The buccal apparatus is - 10 um wide for its whole length. It extends from the upper third of the right side of the ventral surface, follows the rounded anterior end and continues along the left border. At - 1/2 of the body length, the peristome curves conspicuously down the right side towards the cytostome. In protargol-stained specimens, 63 (60-65) membranelles of the adoral zone (AM) are clearly distinguishable: they extend from the left side of the peristome towards the right side almost completely covering the buccal cavity. The membranelles at the cytostomal level are closer to each other and a short paroral is barely visible on the right side. The nuclear apparatus consists of two spherical macronuclei, 13.8 (8-18) um in diam., and of two small micronuclei, 1-2 um in diam" (1).
"Ciliates of the genus Peritromus Stein 1862 are widespread in marine habitats; they have been reported in Africa, America, Asia, and Europe. Only one fresh-water species, namely P. hydrurum has been reported (Chardez 1983). Peritromus are peculiar among Heterotrichea for their dorso-ventral differentiation, recalling that of the hypotrichs. Carey (1992) in his book on marine interstitial ciliates, reported 10 species but, more recently, Song and Wilbert (1997) synonymized some of these species considering their separation to be based on minor differences" (1).
- A Multidisciplinary Approach to Describe Protists: a Morphological, Ultrastructural, and Molecular Study on Peritromus kahli Villeneuve-Brachon, 1940 (Ciliophora, Heterotrichea). GIOVANNA ROSATI, LETIZIA MODEO, MICHELE MELAI, GIULIO PETRONI and FRANC0 VERNI J. EUKARYOT. MICROBIOL., VOL. 51, NO. 1, JANUARY-FEBRUARY 2004
-
Taxonomy and SSU rDNA-Based Phylogeny of Two Heterotrich Ciliates (Ciliophora, Heterotrichea) Collected From Subtropical Wetlands of China, Including the Description of a New Species, Linostomella pseudovorticella n. sp. Didi Jin, Xuetong Zhao,Tingting Ye, Jie Huang, Alan Warren,
Saleh A. Al-Farraj and Xiangrui Chen. Front. Microbiol., 06 September 2021 Sec. Aquatic Microbiology Volume 12 - 2021 Article 719360 https://doi.org/10.3389/fmicb.2021.719360
Photos / Sounds
What
Rhabdomonas incurvaObserver
peptolabDescription
Rhabdomonas incurva (Fresenius, 1858), 11-15 um in length, from the northernmost edge benthos of the spring-fed freshwater coastal pond at Ocean Dunes Apartments in the Atlantic Double Dunes Preserve. Imaged in Nomarski DIC on Olympus BH2S using SPlan 40 0.70 and SPlan 100 1.25 oil objectives plus variable phone cropping on Samsung Galaxy S9+.
Classification:
Rhabdomonas Fresenius 1858
Order Eutreptiales; Family Astasiaceae
Morphology: Colorless osmotrophic unicells with one emergent flagellum that is mobile throughout its length (unlike the stiff flagellum of Euglena), cylindrical (not flattened), 6 to 8 diminutive helical ‘flutes’ (shallow keels), 10 - 35 μm long, with abundant paramylon bodies mainly as oval loops (links). Cell walls are rigid (Leedale 1967).
Description of Rhabdomonas incurva provided by BioPedia
Rigid euglenid, cell outline cylindrical and curved, dorso-ventrally slightly flattened. Anterior end is rounded and slightly oblique, posterior end of cell is rounded. Cells are about 11 - 16 microns long. The opening of the apical canal is located laterally. With one flagellum which is about the same length as the cell, sometimes stretched straight out when the cell is at rest or after death. The pellicle has 4, 6 or 8 thin longitudinal grooves (spaced about 0.5 - 2 microns wide). Some cells have very delicate grooves (hard to see), other cells have distinct grooves. A nucleus was observed in or slightly posterior to the middle of the cell. A contractile vacuole is located in the anterior third either near the centre or to one side of the cell. Some cells contain two large ovale paramylon grains in the aneroir part of cell and some small roundish in the posterior part of cell, other cells contain also a few cylindrical paramylon grains in the middle of cell. During swimming cells rotate around the longitudinal axis.
From: Fresenius, G. (1858). Beiträge zur Kenntniss mikroskopischer Organismen. Abhandlungen der Senckenbergischen Naturforschenden Gesellschaft 2(2): 211-242, plates 10-12.
Rhabdomonas incurva Fresen. In standing water with Conferven, Vallisneria etc. in the botanical garden.
Body 1/60 —1/50 mm long, 1/200- 1/150 mm thick, elongated-cylindrical, slightly sickle-shaped curved, slightly thicker at the front end, almost truncated there, along the length usually three clearly prominent lines striped, usually only in the front.Half filled with pale greenish bubbles or kernels. Just moving forward with full or half rotations around the longitudinal axis. Movement organ one without Iodine barely detectable thread, about the length of the body, even one and a half times as long. With slight differences in shape and size, I still like this species occurred in a few other places; so e.g. also quite often in a sand pond near the Ziegelhütte near Sachsenhausen in the company of numerous Pediastrians etc. These specimens were only 1/75- 1/66 mm long.
Photos / Sounds
What
Bursaria truncatellaObserver
kenkneidelDescription
205 microns, length 448 microns in second photo
Photos / Sounds
What
Genus BursariaObserver
kenkDescription
Collected from a freshwater pond. Very large. I estimate the long axis to be approximately 750µm (field of view is 1000µm)
Photos / Sounds
What
Bursaria truncatellaObserver
peptolabDescription
Bursaria truncatella from our local fresh water Town Pond-sample taken from surface scum. I found two individuals measuring 300 um one day and a cyst the next. Their favorite meal seemed to be the abundant Paramecium bursaria also present in the sample.
Imaged in Nomarski DIC using Olympus BH2S under SPlan 20x objective plus variable phone cropping on Samsung Galaxy S9+.
From: https://en.wikipedia.org/wiki/Bursaria_(ciliate)
Bursaria is a genus of ciliates in the class Colpodea. They are relatively large and feed on other protists in freshwater habitats.
The cell body of Bursaria is scoop-shaped, and can be up to 1 mm long. Their oral region is crescent-shaped, and there is a band of membranelles (compound structures composed of multiple cilia) leading into the mouth. They live in the plankton of freshwater environments and ingest other protists, including algae and other ciliates.[1] Bursaria are the largest-sized colpodean ciliates.[2]
Systematics
Bursaria is the sole genus in the family Bursariidae.[2] The genus was originally described by O. F. Müller in 1773, with B. hirundinella as the type species.[3] It was formerly classified as a heterotrich, but is now classified as a member of the class Colpodea, based on the development of its oral structures (stomatogenesis) and its ultrastructure. [2]
From:https://www.nies.go.jp/.../morpho/ciliopho/bursaria.htm
Bursaria O.F. Muller, 1773
Class Kinetofragminophora: Subclass Vestibulifera: Order Bursariomorphida
Suborder Bursariomorphina Fernandez-Galiano, 1978: Family Bursariidae Dujardin, 1840
The body is approximately ovoid in shape with the anterior truncated and the posterior broadly truncated. The dorsal surface is convex and the anterior half of the flattened ventral surface is divided by a wide slot which leads to the deeply invaginated peristomial cavity. The peristomial cavity opens anteriorly and is divided internally by a longitudinal fold; the cytopharynx is bent towards the animal's left. The membranelles wind clockwise around the opening of the peristomial cavity on the anterior edge and the body and dip into the cavity on the left side. The body is completely and uniformly covered in longitudinal rows of cilia. The macronucleus is band-like and there are many micronuclei. There are many contractile vacuoles which are distributed along the lateral and posterior edges of the body.
Photos / Sounds
What
Muranothrix felixObserver
peptolabDescription
A muranotrich anaerobe, Murathrix felix Méndez-Sánchez, Pomahač, Rotterová, Bourland and Čepička 2023, from the benthos of a channel connecting estuary Napeague Bay with Fresh Pond. The salinity of the channel is 30%. Imaged in Nomarski DIC on Olympus BH2 using SPlan 40 0.70 and SPlan 100 1.25 oil immersion objectives plus variable phone camera cropping on Samsung Galaxy S9+. The cell measures 120 um in length. The number of macronuclear nodules is 7-8 which is a major character and is consistent with M. felix (Daniel Méndez-Sánchez, personal communication). "Muranothrix felix sp. nov. can be easily distinguished from M. gubernata by having fewer adoral membranelles (26–33 vs. 40–56) and macronuclear nodules (6–14 vs. 15–33; Rotterová et al., 2020" (1). However it must be said that "Muranotrichean diversity in brackish and marine anoxic habitats is likely much greater than current reports would suggest" (1).
"Recently, three new classes of anaerobic ciliates, Odontostomatea, Muranotrichea, and Parablepharismea, have been erected based on molecular and morphological data (Fernandes et al., 2018; Rotterová et al., 2020). According to 18S rRNA gene
analyses, these three classes are closely related to Armophorea (Metopida+Clevelandellida) and, possibly, Cariacotrichea, forming a clade of exclusively obligate
anaerobes (Rotterová et al., 2020)" (2).
"The recently erected class Muranotrichea currently comprises two monotypic genera of anaerobic ciliates (respective type species Muranothrix gubernata and Thigmothrix strigosa), inhabiting anoxic marine or brackish environments, characterized by slender cells with a rightward-spiraling anterior neck region, and invariably coated with prokaryotic ectosymbionts (Rotterová et al., 2020)"(1,2).
Méndez-Sánchez et al 2023 describe the new species as follows: "Cells 80–140×14–21 μm in vivo. Cells elongated, narrowly the postoral body part broadly ellipsoidal, “pin-shape”. Slender anterior neck region spiralized rightward approximately 360°. Cortex flexible, with longitudinal furrows. Surface of the cortex coated with rod-shaped bacteria (ca. 2 μm long) oriented transversely between ciliary rows. Cytoplasm with sparse globular inclusions up to 5 μm in diameter, food vacuoles contain prokaryotes, diatom frustules. Contractile vacuole terminal. Macronuclei comprise 6–14 spherical to ellipsoidal nodules (about 10 μm), each with several nucleoli, clustered in mid-body region, one single spherical micronucleus (ca. 2.7 μm in diameter) among macronuclear nodules. Swims rapidly, intermittently reverses course. Somatic ciliature composed of ordinarily spaced dikinetids, only anterior basal body ciliated, cilia about 10 μm long, arranged in 10–12 narrowly spaced somatic kineties, lying on more or less straight longitudinal furrows. Bristle-like somatic cilia (ca. 10μm long) interspersed at regular intervals along kineties and perpendicularly oriented to body margin. Caudal cilia intermittently converge in an inverted cone arrangement surrounding stiffened much longer (34–65μm) coherent caudal cilia. Peristome elongated, inverted keyhole shaped, spiralizes around anterior neck region. Buccal cavity more or less equatorial. Buccal ciliature comprises long paroral membrane parallel to adoral zone of membranelles, both spiralize rightward about 360° extending almost to anterior end of cell. Adoral zone extends from buccal cavity to terminate subapically, comprises 26–33 membranelles, all of similar size (ca. 5 μm long). Paroral membrane long reverse J-shape, originates on left posterior wall of buccal cavity, borders its posterior margin, and extends anteriorly in peristome, occupies about 90% of adoral zone length, bears long cilia (about 15 μm) resembling a “velum” or “sail” (1).
- Morphology and phylogenetic position of three anaerobic ciliates from the classes Odontostomatea and Muranotrichea (Ciliophora). Daniel Méndez-Sánchez, Ondřej Pomahač, Johana Rotterová, William A. Bourland and Ivan Čepička. J. Eukaryot. Microbiol. 2023;00:e12965. https://doi.org/10.1111/jeu.12965
- Rotterová, J., Salomaki, E., Pánek, T., Bourland, W.A., Žihala, D., Táborský, P. et al. (2020) Genomics of new ciliate lineages provides insight into the evolution of obligate anaerobiosis. Current Biology, 30, 1–14.
Photos / Sounds
What
Epistylis plicatilisObserver
peptolabDescription
Epistylis plicatilis EHRENBERG, 1838 from the northernmost edge benthos of the spring-fed freshwater pond at Ocean Dunes Apartments in the Atlantic Double Dunes Preserve. The finely striated zooids measure 110 um in length and we can see the very slim funnel shape and the characteristic annular contraction enveloping the base of the stem. The micronucleus in this colony is horseshoe-shaped. Dr. Martin Kreutz reported a population with kidney shaped macronuclei (1). Like Dr. Kreutz, I did not identify the micronucleus. Imaged in Nomarski DIC on Olympus BH2S using SPlan 40 0.70 objective and SPlan 100 1.25 oil objective plus variable phone camera cropping on Samsung Galaxy S9+.
Epistylis is one of the most speciose genera of Peritrichia, with more than 200 nominal species (Corliss 1979; Lu et al. 2020). Its members are colonial with a non-contractile stalk, well-defined peristomial lip, oral ciliary rows that make less than two circuits before entering the infundibulum, and a transverse silverline system (Corliss 1979; Kahl 1935; Kühner et al. 2016).
"The zooids of Epistylis plicatilis are quite large with a length of about 100 µm and they are located in the periphery of the colony, which gives them an umbrella-shaped appearance. An important identification feature is the shape of the contracted zooids). The front end is snout-shaped, while the posterior half is annulated and extends over the stalk. The macronucleus can be horseshoe-shaped or kidney-shaped and lies transversely below the peristome. In my population the macronucleus was always kidney-shaped. I could not identify the spherical micronucleus. It should not be attached to the macronucleus, but should be shifted towards the peristome. The non-contractile stalk is thick and has a longitudinal striation, which can vary in intensity)" (1).
"Zooids 90–120 µm long, width 25–50 µm, extended zooids slenderly funnel-shape, contracted zooids with snout-shaped anterior end, posterior part annullated. Macronucleus kidney-shaped to horseshoe-shaped, horizontal in anterior half micronucleus located between peristome and macronucleus, one contractile vacuole near peristome, pellicle with about 117 transverse striae. Stalk non-contractile with longitudinal striation, width 7–18 µm, dichotomously branched colonies up to 4 mm long, zooids are located in periphery of colony" (1).
"Epistylis plicatilis EHRENBERG, 1838 (Fig. pp. 687, 17, 18). Gr. around 100 um· Very slim funnel-shaped; during contraction transversely folded at the back and crossing the base of the stem. Shape very slim (3-4: 1), according to SEHRÖDER quite in the front slightly bell-shaped, funnel-shaped according to EHRENBERG, finely striated. Nucleus horseshoe-shaped, transverse in front. c. V. near the peristome colony with short main and long terminal stems (according to EHRENBERG)" (2).
- https://realmicrolife.com/epistylis-plicatilis/
- A. Kahl; [I: Wimpertiere oder Ciliata (Infusoria) 4. Peritricha und Chonotricha]. Germany:Jena Verlag von Gustav Fischer., 1935
Photos / Sounds
What
Genus SpirostomumObserver
eyekosaederDescription
Magnification: 40x
Microscope: Swift SW380T
Camera: iPhone X
They appeared in masses in our garden pond.
Photos / Sounds
Observer
amayakanDescription
Video: https://youtu.be/DpILCfwFwFA
Photos are snapshots of the same individual featured in the video
Size: 76 µm in length, 36 µm in width
Site of sample collection: A stream full of algae near the South Pond, Katsurashima Ryokuchi (a freshwater habitat), Sendai, Japan
Date of collection: August 27th, 2022
Weather: Cloudy
Water temp.: 25.4°C
pH 7.0
Date of observation: August 28th, 2022 (the collected sample in a plastic container was left near a window out of direct sunlight at room temperature until observation)
Bright-field observation using a Wraymer microscope (model BX-3500TL, Osaka, Japan) equipped with a Floyd-2 HDMI ethernet digital camera (Wraymer, Osaka, Japan). The accuracy of the scale bar was confirmed by using a stage micrometer glass slide (1 div. = 10 µm; Wraymer, Osaka, Japan) at each magnification.
Photos / Sounds
What
Genus LoxodesObserver
amayakanDescription
Video: https://youtu.be/Pr94XvqwciI
Photos are snapshots of the same individual featured in the video
Cell size: 204 µm in length
Site of sample collection: A wetland (rich in Spirogyra) near the pond, Takamori Ryokuchi (a freshwater habitat), Sendai, Japan
Date of sample collection: July 2nd 2022
Weather: Sunny
Water temp.: 28.6°C
pH 7.2
Date of observation: July 2nd 2022
Bright field observation using a Wraymer microscope (model BX-3500TL, Osaka, Japan) equipped with a Floyd-2 HDMI ethernet digital camera (Wraymer, Osaka, Japan). The accuracy of the scale bar was confirmed by using a stage micrometer glass slide (1 div. = 10 µm; Wraymer, Osaka, Japan) at each magnification.
Photos / Sounds
What
Dileptus viridisObserver
bdstaylorDescription
A green species of Dileptus was found and described by C.G. Ehrenberg in Berlin, in 1833. It has not been redescribed since then. The species in Ottawa's Mer Bleue Bog is a true Dileptus, with a macronucleus made up >50 scattered nodules (see second image). Toxic extrusomes (Type I, per Vdacny & Foissner) are rod-shaped and 4-5 μm long (see third and fourth images). Type II extrusomes were not observed, but are probably present. The ciliate occurs throughout the summer at one location, and often blooms very abundantly in September.
Photos / Sounds
What
Genus CymbellaObserver
amayakanDescription
Video: https://youtu.be/oTtxnNvHD0E
Size: see scale bars
Site of sample collection: South Pond, Katsurashima Ryokuchi (a freshwater habitat), Sendai, Japan
Date of collection: Aug. 27th, 2022
Weather: Cloudy
Water temp.: 25.4°C
pH 6.8
Date of observation: August 27th, 2022
Bright field observation using a Wraymer microscope (model BX-3500TL, Osaka, Japan) equipped with a Floyd-2 HDMI ethernet digital camera (Wraymer, Osaka, Japan). The accuracy of the scale bar was confirmed by using a stage micrometer glass slide (1 div. = 10 µm; Wraymer, Osaka, Japan) at each magnification.
Photos / Sounds
What
Avelia multinucleataObserver
peptolabDescription
Avelia multinucleata Dragesco 1999 from a small tidal pool in the salt marsh at the edge of estuary Acabonac Harbor at Landing Lane. These huge ciliates, karyorelectids belonging to the family Geleiidae, measure 2000-3000 um in length and are darkly pigmented brown by pigmentocysts present in the ectoplasm and the endoplasm. The contractile body is divided into three parts: a thinner apical end terminating in a curved beak at the apical end and a broader main body with a blunt slightly narrowed posterior end. The mouth is situated in the narrowed apical segment, in this species at the base of the narrowed apical segment where it joins the main body (as depicted by Dragesco 1999). There are two nuclear groups in this individual- one is clearly visualized and seen to possess three spherical macronuclei and three smaller spherical micronuclei. The second nuclear group is largely obscured by dense pigmentation but can be seen to possess two spherical macronuclei. The fact that there are more than one nuclear group and more than two macronuclei is indicative of the diagnosis Avelia macronucleata (Dragesco 1999).
Imaged in Nomarski DIC on Olympus BH2S using SPlan 10 0.30 and SPlanapo 20 0.70 objectives and SPlan 100 1.25 oil objective with oiled condenser and variable phone camera cropping on Samsung Galaxy S9+.
"Diagnosis: Avelia multinucleata has the characteristics of the genus: gigantism (1100 to 3000 um in vivo), massive cylindrical body, provided with a thinner apical region and with a very short caudal point. The mouth, rather small in size, is located approximately 150 um from the apical tip. The general appearance is quite that of the type species Avelia martinicense (NOUZAREDE 1975) but is distinguished by the presence of four to sixteen macronuclei (instead of the usual two) and two to five micronuclei. The oral infraciliature is that which characterizes the genus. Etymology: "multinucleata" refers to the presence of a number of macronuclei greater than two. Sampling location: Etang de Thau (fine sand, depth 50 cm)" (1).
"Description: In vivo, this giant ciliate appears very contractile and very mobile, sometimes winding into a circle- Fig. 117 shows the actual shape and size of the cells, seen in a PETRI dish, in the middle of grains of sand). The body is more or less flattened laterally, depending on the state of contraction of the ciliate. Between the slide and the coverglass, A. multinucleata is always a little contracted and its color is dark brown, almost black. This color is due to a multitude of brown pigmentocysts arranged both in the interkinetic ectoplasm and in the endoplasm, preferably around the nuclear assemblies. These pigmentocysts are of two kinds: the largest have an ovoid shape and measure 1.0 to 1.6 um in length, the smallest are at the limit of perception (< 0.2 to 0.3 µm). The endoplasm is loaded with varied inclusions, blackish spheres of two to five μm and transparent disc-shaped platelets reaching 2.5 μm. The ciliate has shown itself to be very resistant to manipulation, in living organisms it supports micro-compression. This artifice allows us to perceive the rosettes very well. nuclear cells and numerous vacuoles. The mouth can be observed at length in living organisms. It can open considerably, the oral slit transforming into a circle. In the center of the mouth opening, we always observe a large mass of pigmentocystes. The cilia of the adoral region are not very long and remain barely visible. On the other hand, the powerful
paroral ciliature is spectacular, its eyelashes beating the water, in synchronism, at the manner of the membranes of the so-called upper cilia" (1). "In Avelia, the buccal infraciliature is made up of an adoral row that is formed by short polykineties of three dikinetids that are connected between them, also different from the pattern that is described for the family" (2).
"The nuclear device is difficult to study, because impregnations with protargol make the trunk endoplasm so dark that the nuclear rosettes are practically indistinguishable. Avelia multinucleata being one of the extremely rare species provided with several nuclear assemblies, we studied some 150 specimens, either on the alive (with strong microcompression) or after fixation with SAN-FELICE and staining by the FEULGEN nucleate reaction. Examination of Table 6 and Figures 144 and 145, show that the populations of Sete have two to five rosettes (nuclear sets), made up of four to 16 macronuclei and two to five micronuclei, (average: two to three rosettes, four to six macronuclei, two to three micronuclei). The dimensions of the macronuclei are larger than in the most Geleiids: length from nine to 20 μm, width six to 19 μm. The micronuclei are ovoid and measure 2-4 um long. The macronuclei do not present large protein spheres (as in most Geleiides) but numerous vacuoles, not very apparent, and a few
DNA-rich chromocenters" (1).
"New definition of Geleiidae: The geleiids are very large ciliates (200–5000 μm) with a cylindrical body and an apical region that is slightly tapered, often in beak form. The oral field is subapical and small in relation to the body length. The geleiids are characterized mainly by a paroral organization, i.e. formed by one intrabuccal kinety that is made up of 1–2 dikinetids, and an extrabuccal row, which can either be made up of long polykineties (e.g. 15 in Avelia) or by only one kinety (as in Gellertia). The adoral zone is very diverse between the genera, as it can be formed by polykineties (e.g. Avelia, Corlissina and Geleia) or by only one row of dikinetids (Gellertia and Parduczia). The somatic and buccal kineties present many argentophilic fibres. The geleiids have different pigment granules in the cortex and cytoplasm and are, in most cases, quite contractile. The nuclear apparatus consists of two macronuclei and one micronucleus" (2).
"Genus Avelia NOUZAREDE 1977 It was in 1975 that NOUZAREDE created the genus Avela genre (dedicated to Professor M. AVEL)
to describe the type species Avela martinicense, found in the coralligenous sands of Martinique. The diagnosis of the new genus describes, with precision, the general form and the arrangement of the mouth but we think that certain details of the oral infraciliature oral do not correspond to reality. Unfortunately the Avela genus existed already, designating a Lepidoptera. NOUZAREDE (1977) therefore had to create a new name for his genus from Martinique, the "nomen novum" Avelia (synonymous with Avela of 1975). Improved diagnosis: The genus Avelia NOUZAREDE 1977 concerns very large geleiids, very elongated in shape, whose body is made up of three parts: a rather massive trunk, a small caudal point and a long, fairly thin, carrying in its apical half a small slit mouth. Oral infraciliature oral is made up of an adoral kineties comprising groups of three dikinetides, linked together. A "dense fibrillar zone" separates the adoral kineties from two peri-oral somatic kineties. In the paroral region, we find intraoral kineties as well as a series of long polykineties, consisting of dikinetids and having the same morphology as the adoral polykineties of Geleia. The nuclear apparatus comprises either two macronuclei framing a micronucleus (as in the very large majority of Geleiides), i.e. several rosettes each containing
sets of two to four macronuclei and one micronucleus" (1).
- Revision des Geleiides (Ciliophora, Karyorelictea) Jean DRAGESCO. Stapfia 66 delivered on : 26.11.1999
- Description and phylogenetic position of Corlissina maricaensis gen. nov., sp. nov. (Karyorelictea, Geleiidae), a novel interstitial ciliate from Brazil, with redefinition of the family Geleiidae. Pedro H. Campello-Nunes, Noemi Fernandes, Martin Schlegel and Inácio D. Silva-Neto. International Journal of Systematic and Evolutionary Microbiology (2015), 65, 4297–4308
Photos / Sounds
What
Arcella spectabilisObserver
bdstaylorDescription
A shelled amoeba, with close views of the crenulated aperture.
Photos / Sounds
Observer
davidfbirdDescription
From a small stream coming out of a culvert by the roadside. Very sluggish, with a thick layer of algae on the surface among the reeds. I was surprised to see that essentially 100% of the phytoplankton were of one very active type. Cell diameter 11-16 microns. Globular, with a single pyrenoid and a red stigma. Four flagella. Carteria pseudoglobosa? Just guessing. I'm not even sure that it is Carteria.The stigmata are mostly hidden as it is strongly phototactic and turns toward the light source. And it is hard to make out the flagella in my microscope. Note that the gif of the plankton at low magnification is of raw water, unconcentrated.
Photos / Sounds
What
Cristatella mucedoObserver
jonnytosteDescription
found in shallow stream flowing into Homer lake.
Photos / Sounds
What
Genus MallomonasObserver
amayakanDescription
Video: https://youtu.be/gPAve5bsw8U
Photos are snapshots of the same individual featured in the video
Size: 39 µm in length (excluding the spines and flagellum)
Site of sample collection: Pavilion, Katsurashima Ryokuchi North Pond (a freshwater habitat), Sendai, Japan
Date of sample collection: July 16th, 2022
Weather: Rainy
Water temp.: 21.3°C
pH 6.2
Date of observation: July 16th, 2022
Bright-field observation using a Wraymer microscope (model BX-3500TL, Osaka, Japan) equipped with a Floyd-2 HDMI ethernet digital camera (Wraymer, Osaka, Japan). The accuracy of the scale bar was confirmed by using a stage micrometer glass slide (1 div. = 10 µm; Wraymer, Osaka, Japan) at each magnification.
Photos / Sounds
What
Genus TrithigmostomaObserver
dgborinDescription
Sample taken on 2024-07-20.
Different specimens: single, dividing, conjugating.
Photos / Sounds
Observer
peptolabDescription
Jenningsia macrostoma (Ekebom et al., 1996) Lee, Blackmore and Patterson 1999 from the northernmost saprobic edge benthos of the spring-fed freshwater coastal pond at Ocean Dunes Apartments in the Atlantic Double Dunes Preserve. The sampling site is just 200 meters from the edge of the Atlantic Ocean.
Imaged in Nomarski DIC on Olympus BH2S using SPlanapo 20 0.70, Splanapo 40 0.95, and SPlan 100 1.25 oil objectives plus variable phone cropping on Samsung Galaxy S9+.
The cells are highly metabolic and measure up to 130 um when fully extended. There are fine pellicular striations in an s-helix configuration. There is a single thick emergent flagellum. Two large ingestion apparatus rods are apparent along with a curved refractile cytoskeletal arc arising from the right rod and curving towards the anterior of the cell. There is a large ellipsoid macronucleus with several large nucleoli in the posterior part of the cell.
"Jenningsia macrostoma (Ekebom et al., 1996) Lee, Blackmore and Patterson, n. comb. This species has been reported with lengths from about 64 to 114 µm. The body is anteriorly narrowed and posteriorly rounded. It is very metabolic and has fine pellicular striations following a S-helix. The flagellar pocket is situated on the left ventral face of the cell. Two flagella are seen in the flagellar pocket, but only one flagellum emerges. The flagellum is slightly shorter than the cell and beats freely. The ingestion apparatus with two well marked rods is strongly developed. A refractile cytoskeletal arc arises from the right rod and curves towards the anterior of the cell. Optical sections through the anterior part of the cell show this as a short curving structure extending from near the front pole of the cell to near the anterior end of the ingestion rods. The nucleus is situated in the posterior part of the cell. Refractile granules are randomly distributed inside the cell. Rod-shaped muciferous bodies lie alongside pellicluar striations. Cells glide with a squirming movement. Less common than J. fusiforme" (1).
"This species has been described as Peranema macrostoma from marine sites at tropical and subtropical Australia, Brazil (Larsen and Patterson, 1990; Ekebom et al., 1996; Lee and Patterson, 1999). Like Peranema fusiforme, it has been known that P. macrostoma has a short, curved recurrent flagellum which is tightly pressed to the cell surface (Ekebom et al., 1996). Figure 4h was taken from Ekebom et al. (1996; Fig. 4e). In this figure, the recurrent flagellum was indicated by an arrow, but the arrow indicates the curving element of the intracellular cytoskeleton. Only one flagellum emerges from the anterior opening canal as there is no recurrent flagellum emerging from the slit-like opening of the flagellar pocket (Fig. 4k)" (1).
"Jenningsia and Peranemopsis were created by Schaeffer (1918) and Lackey (1940) respectively to contain peranemid flagellates having one emergent flagellum and flexible body with pellicular striations. They are distinguished from the genus Peranema by having one flagellum, other species having a recurrent flagellum lying in a groove on the ventral face of the body (Fig. 4g). Peranema fusiforme and P. macrostoma have been described with a short, curved recurrent flagellum which is tightly pressed to the cell surface (Larsen, 1987; Larsen and Patterson, 1990; Ekebom et al., 1996). Figure 4a of Peranema fusiforme was taken from Larsen and Patterson (Larsen and Patterson 1990; Fig. 24e) and figure 4h is of Peranema macrostoma and from Ekebom et al. (Ekebom et al., 1996; Fig. 4e), respectively. In these figures, the structure that was interpreted as the recurrent flagellum was indicated by arrows. Our present observations of both species indicate that the structure is a previously undescribed element of the cytoskeleton associated with the ingestion apparatus. As seen in figures 4d and 4k, only one flagellum emerges from the anterior opening canal (arrows). We are therefore of the view that both of these species lack an emerging recurrent flagellum. As the genus Jenningsia was described and distinguished from Peranema on the basis of the absence of the second flagellum, we believe these two species are most appropriately transferred to that genus. Peranemopsis was described by Lackey (Lackey, 1940) also as a peranemid with a single emergent flagellum. It was described without reference to Jenningsia" (1).
- Australian records of two lesser known genera of heterotrophic euglenids – Chasmostoma Massart, 1920 and Jenningsia Schaeffer, 1918 W.J. Lee, R. Blackmore and D.J. Patterson. Protistology 1, 10-16 (1999).
Photos / Sounds
What
Family ColepidaeObserver
manuel_helsinkiDescription
In a sample of water from Töölö Bay, Baltic Sea
Photos / Sounds
What
Speckled Blister Lichen (Viridothelium virens)Observer
davidfbirdDescription
On the bark of a linden tree, along with patches of Graphis scripta. Photobiont is Trentepohlia - this very humid valley is rich with pure patches of Trentepohlia. The small piece of loose bark I found had no apothecia, but did have two pycnidia. Conidia were hyaline, bacilliform, about 4-5 microns long and 1.2 microns wide.
Photos / Sounds
What
Genus TrithigmostomaObserver
dgborinDescription
Sample taken on 2024-07-20.
Slowing solution 0.5% Methyl cellulose
Photos / Sounds
What
Genus FrontoniaObserver
crseaquistDescription
Water sample (freshwater) was taken on 07/20/2024 using a turkey baster..
Photos / Sounds
What
Eastern Cicada-killer Wasp (Sphecius speciosus)Observer
mnold1Description
Last image shows the Eastern Cicada-killer on the pile of dirt and gravel excavated while digging its nest. (impressive pile!)
Photos / Sounds
Observer
davidfbirdDescription
From a macrophyte bed on the lakeshore of a eutrophic lake. No sheath, aerotopes or heterocytes. Motile, 5-5.5 x 8.5 µm W x L. Cells constricted in the middle and at crosswalls. Terminal cells slightly swollen, rounded.
Photos / Sounds
What
Life (Life)Observer
goldfjnchDescription
40x objective
sample from fresh water pond
image 4 is sped up 400%
image 5 is sped up 5000%
Photos / Sounds
What
Spirulina majorObserver
davidfbirdDescription
From a stagnant stormwater pond. Coil length 2.5-3.2 µ, coil diameter 3.6-4.1, cell width 1.8 µ, filaments isolated, actively motile. The gif is real-time.
Photos / Sounds
What
Copepods (Subclass Copepoda)Observer
pinefrogDescription
Collected from a permanent freshwater woodland pond.
Photos / Sounds
What
Common Raccoon (Procyon lotor)Observer
mnold1Description
4 young raccoons (siblings?) foraging together in a relatively tight group - fun to watch!
Photos / Sounds
What
Order CocconeidalesObserver
kenzieb87Description
10-40x Objective
Freshwater
Dyes: Bismarck Brown, Neutral Red
AmScope B120C Microscope
MD100 AmScope Camera
I am still new to microscopy, staining, and identifying microscopic organisms in general. Any tips for bettering my observations would be greatly appreciated. I also appreciate when people explain their identifications.
Photos / Sounds
What
Genus OphryoglenaObserver
davidfbirdDescription
From a surface sample collected on the shoreline, where there was an accumulation of pollen. Ovoid but very flexible ciliates with a figure-of-6 spiral opening to the mouth, watchglass-shaped lens over the X-shaped pigment spot, large lateral vacuole and baguette-shaped nucleus. These observations are of a phase where they tend to rotate in place. (I'll post separately an entry for the frenetic run-about-madly phase that I just recognized to be this same species. Link: https://inaturalist.ca/observations/223984696). They often develop an indentation at the mid-section, with sometimes an odd little projection. The still shots are screenshots from video footage, to show some of the morphological contortions they exhibit.
Photos / Sounds
Observer
someplantDescription
Magnification of photos: 600×, 600×, 600×, 600×, 600×
Habitat: Myriophyllum and Spirogyra growing at the bottom of a reservoir.
Photo taken with a Celestron PentaView Digital Microscope. According to their website, the FOV (i.e. the diagonal width) at 600× is 100 µm.
https://www.celestron.com/blogs/knowledgebase/what-is-my-digital-microscopes-field-of-view
The cell body "wobbles" by rocking from side to side while moving. I could see one flagellum, which was trailing.
Photos / Sounds
What
Life (Life)Observer
crseaquistDescription
Water sample (freshwater) was taken on 07/10/2022 using a coffee filter to enrich for microorganisms.
Photos / Sounds
What
Closterium lineatumObserver
bennett-sDescription
Cow horn shaped diatom-like organism with longitudinal striations from end-to-end.
The site is a sphagnum bog that abuts a pond.
Photos / Sounds
What
Diplophrys archeriObserver
mnold1Description
The 1st and 2nd photos are enlargements of sections of the colony when first encountered, i.e, when the cells were only loosely associated. Photos 3 through 10 capture the relatively rapid aggregation of the cells into a tight cluster; aggregation appeared near maximum within 1.5 minutes after the start of the observation. I suspect exposure to the focused illumination from the scope somehow triggered the aggregation.
Amazing to witness! Interesting (and fast!) signaling biochemistry at play here! ...or is this similar to the coordinated flight of bird flocks, relying on "visual" cues?
Water sample collected from a vernal stream, near moss-covered rocks and leaf-covered bottom; water depth 4-5 inches with very little flow. Air temp 55F
Observer
gpmatthewsDescription
Gastronauta membranaceus
Microscope: Leitz Dialux
Ocular: 10x GF Periplan
Objective: 40/0.70 NPL Fluotar ICT (DIC)
Sample from garden pond
Flash
What
Diatoms (Class Bacillariophyceae)Observer
mnold1Description
Mag. 400x
Fragment of a tube-dwelling Navicula sp. colony? (Looks more like Navicula than Cymbella and Frustula, both of which have tube-dwelling varieties). I count 7 cells in this package... 8 would make more sense if the number is linked to binary replication. Also interesting is the size variation; e.g. in the 3rd panel from the left one sees a small cell in the foreground with a mix of much larger cells behind (makes one wonder about the replicational relationship among the cells... perhaps there is none).
- On 3/29/2022 a water and moss sample were taken from the submerged section of a boulder in the Billings-Avery Brook. Air temp. 38F
Photos / Sounds
What
Genus SpirulinaObserver
maricel-patinoDescription
Found in water collected from a rice canal.
What
Diatoms (Class Bacillariophyceae)Observer
laneallenDescription
All specimens from the Colorado Front Range.
Scale Bar = 10 µm.
Photos / Sounds
What
Sac Fungi (Phylum Ascomycota)Observer
maricel-patinoDescription
On an orchid leaf.
Photos / Sounds
What
Euglenoids (Phylum Euglenozoa)Observer
valveDescription
freshwater constructed wetland.
Photos / Sounds
What
Genus DinobryonObserver
amayakanDescription
Video: https://youtu.be/sfbkRjOaZI0
Photos are snapshots of the same individual in the video
Cell size: 48 µm in length (excluding flagella)
Site of sample collection: Pavilion, Katsurashima Ryokuchi north pond (a freshwater habitat), Sendai, Japan
Date of sample collection: March 26th 2022
Weather: Cloudy
Water temp.: 9.0°C
pH 6.4
Date of observation: March 27th 2022 (the collected sample in a plastic container was left near a window out of direct sunlight at room temperature until observation)
Bright field observation using a Wraymer microscope (model BX-3500TL, Osaka, Japan) equipped with a Floyd-2 HDMI ethernet digital camera (Wraymer, Osaka, Japan). The accuracy of the scale bar was confirmed by using a stage micrometer glass slide (1 div. = 10 µm; Wraymer, Osaka, Japan) at each magnification.
Photos / Sounds
What
Order CraspedidaObserver
mnold1Description
Mag. 400x
Choanoflagellates (on a filament of Eunotia sp.).... perhaps genus Salpingoeca as seen here https://www.inaturalist.org/observations/85163828 and http://cfb.unh.edu/phycokey/Choices/Amoebae_Flagellates_Ciliates/Choanoflagellates/SALPINGOECA/Salpingoeca_Image_page.html#pic02.
- A pond-side water sample was taken on 12/27/2021 using a 10µ dip net. About 1/4" of ice had to be broken and pushed aside to access the water. Air temp. 36°F.
Photos / Sounds
What
Arthrospira jenneriObserver
mnold1Description
Mag. 400x
Helical cyanobacteria with crosswalls that are difficult to see, but present (indicated by black arrows where they are distinct). Specie: possibly A. jenneri as seen here https://www.researchgate.net/publication/330579181_Detailed_characterization_of_the_Arthrospira_type_species_separating_commercially_grown_taxa_into_the_new_genus_Limnospira_Cyanobacteria.
A pond edge water sample (freshwater) was taken on 10/1/2021 using a 10 micron dip net to enrich for microorganisms.
Photos / Sounds
What
Genus MallomonasObserver
amayakanDescription
Video: https://youtu.be/gAYpJMw-Zp8
Photos are snapshots of the same individual in the video
Cell size: 16 µm in length, 11 µm in width
Site of sample collection: Pavilion, Katsurashima Ryokuchi north pond (a freshwater habitat), Sendai, Japan
Date of sample collection: March 26th 2022
Weather: Cloudy
Water temp.: 9.0°C
pH 6.4
Date of observation: March 27th 2022 (the collected sample in a plastic container was left near a window out of direct sunlight at room temperature until observation)
Bright field observation using a Wraymer microscope (model BX-3500TL, Osaka, Japan) equipped with a Floyd-2 HDMI ethernet digital camera (Wraymer, Osaka, Japan). The accuracy of the scale bar was confirmed by using a stage micrometer glass slide (1 div. = 10 µm; Wraymer, Osaka, Japan) at each magnification.
What
Bdelloidean Rotifers (Subclass Bdelloidea)Observer
plingfactoryDescription
Bdelloid rotifer with a "saddle" out of detritus; several specimens found, always with a "saddle". Seems not yet described in the literature. More on this here:
https://www.plingfactory.de/Science/Atlas/KennkartenTiere/Rotifers/01RotEng/source/Bdelloid_20.html
Photos / Sounds
What
Protozoans (Kingdom Protozoa)Observer
woodilljDescription
- from marine sample (sand and water), collected at shoreline
- objective(s) (x 10): 600x
- approximate dimensions: body: 15 µm, longest axis; flagella: 23 µm
- video on Vimeo
- ID: ELPB-LGT40
- Body apparently rigid and perhaps bilaterally symmetrical.
- Eight ~~nine~~ (?) flagella emerge from spiky/knobby protuberances.
- Organism is transparent and internal structures are not readily apparent in brightfield.
- 'Walks' or glides along using flagella, sometimes pushing off and 'coasting' (see animated GIF #2 or above-linked video).
- Often, flagella not immediately applied to locomotion are waved busily about (see animated GIF #1 or above-linked video).
Photos / Sounds
What
Genus AscomorphaObserver
mnold1Description
Mag. 400x (progression of focal planes)
Pollen? Anyone one know of a handy online reference for identifying the pollen of various plants?
A pond-edge water sample was collected on 6/2/2021 using a 10 micron dip net to enrich for microorganisms. Air temp. 67F
Photos / Sounds
What
Colpoda cucullusObserver
peptolabDescription
Colpoda cucullus O.F. Muller, 1773 from algae-infused water wrung from fallen leaves that spent the winter submerged on my swimming pool cover. Imaged in Nomarski DIC on Olympus BH2S using SPLANAPO 40 0.95 objective and SPLAN 100 1.25 oil objective with slide oiled to the condenser. The cells measure from 80-100 um in length. We can clearly see the two most important characters which differentiate C. cuculus from other species in the genus: the stellate endosome of the spherical macronucleus and the anterior keel with 9-10 indentations.
" 40-110 um long; anterior keel with 8 to10 indentations; 29-34 ciliary grooves; cilia mostly paired; macro nucleus with a stellate endosome; trichocysts rod-form; usually with abundant food vacuoles; in fresh water with decaying plants" (1).
"Body distinctly reniform in shape, dorso-ventrally flattened. Right body edge strongly convex, left body edge concave often appearing as through a bite had been taken from it. A shallow diagonal somatic groove (not easily visible) originating on the dorsal surface travels round left side to entrance of vestibulum on the flattened ventral surface. Ciliation uniform in longitudinal or oblique orientated grooves. Several notches which denote ciliary grooves often visible on preoral part of left body edge. Caudal cilia may be present on some species. There is a horse-shoe shaped arc of closely-set cilia on the right of the vestibular entrance. Single rounded macronucleus with 1, 2 or 3 micronuclei. Single terminal contractile vacuole. Division takes place in thin-walled cysts, thick-walled protective cysts also formed" (2).
"Morphology Length 40-120 um, average about 80 um; broadly reniform, anterior keel with 8-10 indentations; uniform ciliation; buccal cavity with a deep oral funnel starting at a groove near the left side of the body, the buccal cavity leads to a diagonal groove on the dorsal surface (not evident in the figure), buccal cavity ciliated but without membranes or membranelles; 1 spherical macronucleus, exhibiting a stellate endosome; 1 micronucleus; a single terminal contractile vacuole; in the presence of a good food supply, the body is packed with food inclusions and appears very dark; fission only takes place within reproductive cysts" (3).
" Colpoda cucullus, previously considered a "soil" protozoan, is predominantly a vegetation-associated species that is especially adapted, through its ability to encyst and excyst rapidly, to exploit the fluctuating moisture content of the terrestrial environment. Three means of distribution were discovered which explain the ubiquity of this species. Herbivores consume Colpoda cysts while feeding on infested vegetation and deposit them with their feces; the cysts are transported by honey-bees along with pollen grains; and they are also carried through air like pollen. Dew induces excystment and contains sufficient nutritional substances to support profuse growth and reproduction" (4).
- PROTOZOOLOGY By RICHARD R. KUDO. CHARLES C THOMAS • PUBLISHER Springfield, Illinois. 1954. pp 745-6.
- Colin R. Curds "British and other freshwater ciliated protozoa Part I Ciliophora: Kinetofragminophora" Cambridge University Press, 1982
- CILIATED PROTOZOA. An illustrated guide to the species used as biological indicators in freshwater biology HARTMUT BICK. WORLD HEALTH ORGANIZATION GENEVA 1972 pp 64-5.
- Colpoda cucullus: A Terrestrial Aquatic.Jo Anne Mueller, Wayne P. Mueller. The American Midland N